Strong Acids and Hydronium Ion
Video by Janet Gray Coonce, MS
If you plan to view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges.
Review the notes and screen shots after viewing the video.
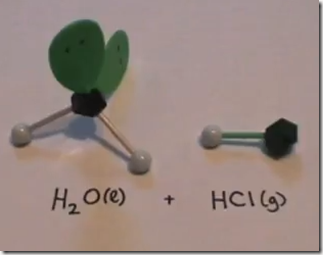
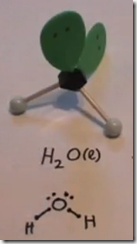
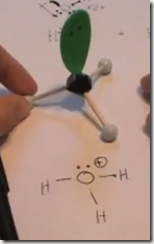
On the Left: Water (H2O) in its liquid (l) physical state is represented by the model and formula on the left. The 2 white hydrogen atoms are bonded to oxygen. There are 2 pairs of unshared electrons on the oxygen. The unshared electrons repel the bonded hydrogen atoms which giving H2O a bent molecular geometry.
On the Right: Hydrogen chloride gas (hydrochloric acid) is represented by the symbol HCl(g). The model consists of a single atom of hydrogen in a covalent bond with chlorine.
Here the Lewis structure of water is drawn. Oxygen has 6 valence electrons. There are 2 lone pairs and 2 pairs of shared electrons which are participating in covalent bonds with hydrogen. As a result of electron sharing, oxygen has a complete octet of valence electrons and hydrogen has a complete duet of valence electrons. The repulsive force of the unshared electrons repel the hydrogens giving the molecule a bent molecular geometry. The molecule is polar with the oxygen end the more electronegative and the hydrogen end more positive.
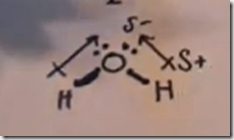
Here the delta(-) and delta(+) notation is used to show the direction of the electronegative forces. Electrons are shared with hydrogen but they are pulled more toward the oxygen than the hydrogen atoms. This separation in charge results in the water molecule being polar with the oxygen pole being more negative—a dipole moment.
Hydrogen chloride gas also has a covalent bond between hydrogen and a very electronegative atom, chlorine.
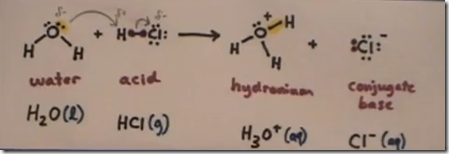
When H–Cl (g) is bubbled into water, hydrogen’s single proton is attracted to and forms a bond with oxygen. The previous unshared pair of electrons is now shared between oxygen and the hydrogen proton. The gives the H3O+ molecule a positive charge. This polyatomic ion is the hydronium ion and forms whenever a strong acid is added to water. The chloride anion (Cl–) forms an ionic bond with the hydronium cation.
To re-cap, the Lewis structures for H2O, HCl, H3O+ and Cl– are drawn to show what happens to the electrons when a strong acid like HCl (g) is added to water in the reaction:
H2O(l) + HCl(g) –> H3O+(aq) + Cl– (aq)
Water accepts a proton from HCL to form the hydronium cation. HCl donates a proton to form the negatively charged conjugate base (chlorine anion). This gives the hydronium molecule a positive charge, the hydronium cation. Strong acids are proton donors and form hydronium cations in aqueous solution.
Often the reaction is abbreviated by ignoring the water.
The net effect of adding a strong acid to water is the donation of a proton, the hydrogen ion. The higher the concentration of hydrogen ions (actually hydronium ions), the stronger the acid.
pH = –log ([H+])
The usual range of pH for aqueous solutions is from 1 to 14. Pure water has a pH of 7. Acidic solutions have a pH < 7 and basic solutions have a pH of > 7.
A strong acid may be defined as a compound which dissociates completely in an aqueous solution into H+ and its conjugate base (anion)–. The hydrogen ion is a proton so you may also see an acid defined as a substance which is a proton donor in an aqueous solution. Remember, the proton (H+) does not exist by itself in water but binds with the water to form hydronium ions as shown in this reaction:
H2O(l) + HCl(g) –> H3O+(aq) + Cl– (aq)
With HCl and all strong acids in aqueous solution, the formation of the hydronium ion may be assumed and the dissociation reactions abbreviated. Here are how several strong acids dissociate in aqueous solution to form hydrogen (actually hydronium) ions:
HCl (aq) —> H+ + Cl–
HBr (aq) —> H+ + Br–
HI (aq) —> H+ + I–
H2SO4 (aq) —> H+ + HSO4–
HNO3 (aq) —> H+ + NO3–
HClO4 (aq) —> H+ + ClO4–
Transcription, notes and comments by James C. Gray MD FACOG
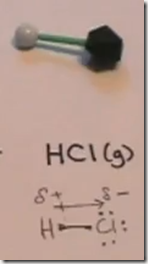
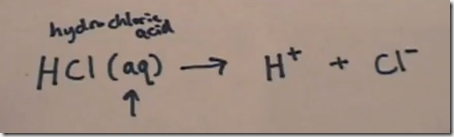
Very nice lectures and videos.
Good effort
The videos are amazing! Truly helpful
Good explanation and try to explain use molecule
Its realy helpful for us & other students
Its very easy to understand &
Very nice and informative video lecture for the students learning first time.i am teacher for A level chemistry