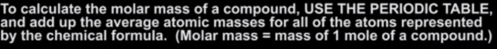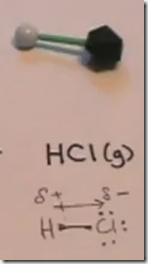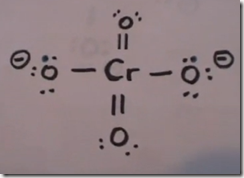by Janet Coonce
In an Olefin (Alkene) Metathesis reaction a carbon to carbon double bond exchanges places with a single carbon to carbon bond.
Video by Janet G. Coonce, MS
If you plan to view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges.
Problem: 77 grams of glucose was allowed to ferment to form ethanol and carbon dioxide. 23.4 grams of ethanol was recovered by distillation. What was the theoretical yield? What was the percent yield? In other words how efficient is my laboratory process in approaching the potential theoretical yield?
,
.
When we write and balance the equation, we find that each mole of glucose, if we were able ferment 100% of 1 mole of glucose to ethanol, should yield 2 moles of ethanol and 2 moles of carbon dioxide:
C6H1206–fermented —> 2CH3CH20H + 2C02
| Atom | Atomic Mass (g/mole) | Number | Molecular Mass | |
| C | 12.01 | 6 | 72.06 | |
| H | 1.01 | 12 | 12.12 | |
| O | 16.00 | 6 | 96.00 | |
| Total | Molar | Mass | 180.18 | g/mole |
| Atom | Atomic Mass | Number | Molecular Mass | |
| C | 12.01 | 2 | 24.02 | |
| H | 1.01 | 6 | 6.06 | |
| O | 16.00 | 1 | 16.00 | |
| Total | Molar | Mass | 46.08 | g/mole |
Video by Janet Gray Coonce, MS
If you plan to view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges.
Review the notes and screen shots after viewing the video.
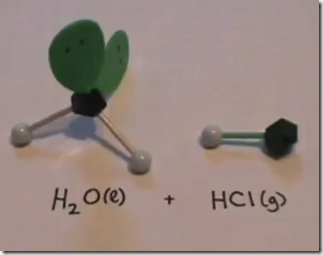
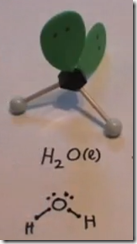
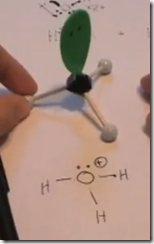
On the Left: Water (H2O) in its liquid (l) physical state is represented by the model and formula on the left. The 2 white hydrogen atoms are bonded to oxygen. There are 2 pairs of unshared electrons on the oxygen. The unshared electrons repel the bonded hydrogen atoms which giving H2O a bent molecular geometry.
On the Right: Hydrogen chloride gas (hydrochloric acid) is represented by the symbol HCl(g). The model consists of a single atom of hydrogen in a covalent bond with chlorine.
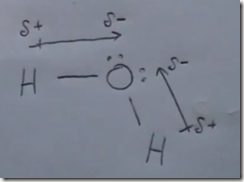
Here the Lewis structure of water is drawn. Oxygen has 6 valence electrons. There are 2 lone pairs and 2 pairs of shared electrons which are participating in covalent bonds with hydrogen. As a result of electron sharing, oxygen has a complete octet of valence electrons and hydrogen has a complete duet of valence electrons. The repulsive force of the unshared electrons repel the hydrogens giving the molecule a bent molecular geometry. The molecule is polar with the oxygen end the more electronegative and the hydrogen end more positive.
Here the delta(-) and delta(+) notation is used to show the direction of the electronegative forces. Electrons are shared with hydrogen but they are pulled more toward the oxygen than the hydrogen atoms. This separation in charge results in the water molecule being polar with the oxygen pole being more negative—a dipole moment.
Hydrogen chloride gas also has a covalent bond between hydrogen and a very electronegative atom, chlorine.
When H–Cl (g) is bubbled into water, hydrogen’s single proton is attracted to and forms a bond with oxygen. The previous unshared pair of electrons is now shared between oxygen and the hydrogen proton. The gives the H3O+ molecule a positive charge. This polyatomic ion is the hydronium ion and forms whenever a strong acid is added to water. The chloride anion (Cl–) forms an ionic bond with the hydronium cation.
To re-cap, the Lewis structures for H2O, HCl, H3O+ and Cl– are drawn to show what happens to the electrons when a strong acid like HCl (g) is added to water in the reaction:
H2O(l) + HCl(g) –> H3O+(aq) + Cl– (aq)
Water accepts a proton from HCL to form the hydronium cation. HCl donates a proton to form the negatively charged conjugate base (chlorine anion). This gives the hydronium molecule a positive charge, the hydronium cation. Strong acids are proton donors and form hydronium cations in aqueous solution.
Often the reaction is abbreviated by ignoring the water.
The net effect of adding a strong acid to water is the donation of a proton, the hydrogen ion. The higher the concentration of hydrogen ions (actually hydronium ions), the stronger the acid.
pH = –log ([H+])
The usual range of pH for aqueous solutions is from 1 to 14. Pure water has a pH of 7. Acidic solutions have a pH < 7 and basic solutions have a pH of > 7.
A strong acid may be defined as a compound which dissociates completely in an aqueous solution into H+ and its conjugate base (anion)–. The hydrogen ion is a proton so you may also see an acid defined as a substance which is a proton donor in an aqueous solution. Remember, the proton (H+) does not exist by itself in water but binds with the water to form hydronium ions as shown in this reaction:
H2O(l) + HCl(g) –> H3O+(aq) + Cl– (aq)
With HCl and all strong acids in aqueous solution, the formation of the hydronium ion may be assumed and the dissociation reactions abbreviated. Here are how several strong acids dissociate in aqueous solution to form hydrogen (actually hydronium) ions:
HCl (aq) —> H+ + Cl–
HBr (aq) —> H+ + Br–
HI (aq) —> H+ + I–
H2SO4 (aq) —> H+ + HSO4–
HNO3 (aq) —> H+ + NO3–
HClO4 (aq) —> H+ + ClO4–
Transcription, notes and comments by James C. Gray MD FACOG
Video by Janet Gray Coonce, MS
Transcription, notes and comments:
This video discusses the chemical structure of fatty acids. What does saturated or unsaturated refer to? What is the difference between a cis and trans isomer?
What is a saturated fatty acid?
This diagram represents the chemical structure of a saturated fatty acid. The long zig-zag, (line-angle formula) component represents a long string of -CH2-CH2-CH2 bonds which terminate in a methyl group (-CH3). This methyl terminal carbon is numbered omega 1 when identifying the carbon molecules in the fatty acid chain. Each vertex in the line-angle formula represents a carbon atom bonded with 2 hydrogens. The omega 1 carbon has 3 bonds to hydrogen (CH3).
This long string of hydrocarbons is very hydrophobic. Hydrophobic means it does not mix easily with water. It will repel water. This is a characteristic of a “fatty” substance. It is non-polar. Carbon and hydrogen have similar electronegativity and each of the hydrocarbon components have a neutral charge so there is no region of negative or positive anywhere along the long hydrocarbon chain. It is non-polar. It is fat soluble (fats are non-polar). It is not water soluble (water is polar).
The terminating carbon on the left side of this illustration is bonded to a hydroxyl group and a double bond to oxygen. This is referred to as a carboxylic acid functional group. The functional end carbon is the alpha carbon. The term fatty acid refers to the fact that this is a carboxylic acid of a long chain hydrocarbon which is fat soluble. In this first illustration there are no double or triple bonds. Each carbon of the chain is saturated with hydrogen atoms. No further hydrogenation is possible because there are no double or triple bonds.
Saturated fatty acids have higher melting points than unsaturated fatty acids. Animal fats are mostly saturated and are solid at room temperature (lard).
What is an unsaturated fatty acid?
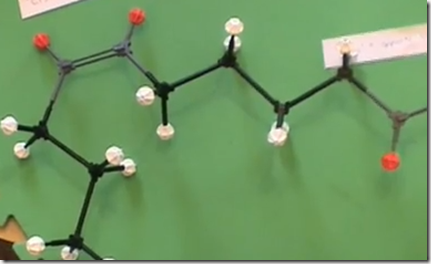
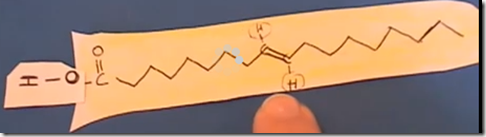
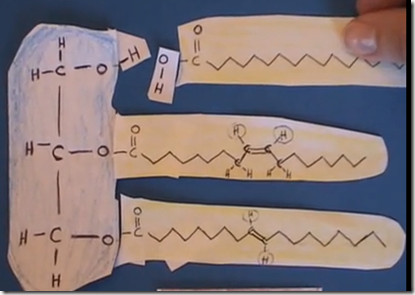
This is the structure of cis-oleic acid. Cis-oleic acid is the isomer which is found naturally in olive oil. In this fatty acid there is a double bond between carbon 9 and 10 counting from the CH3 terminal carbon (omega). The carbon atoms connected to each other by the double bond are unsaturated with hydrogen. They each only have one bond with hydrogen. In this example, there is only one double bond between omega carbon 9 –10. This is an omega-9 monounsaturated fatty acid. If there were more than one double bond in a fatty acid it would be referred to as a polyunsaturated fatty acid.
Unsaturated fatty acids are not as stable as a saturated fatty acid. The double bond between carbons in a hydrocarbon chain invites the formation of a fatty acid radical which can react with oxygen in the process known as rancidification. This can lead to formation of ketones and aldehydes which are responsible for the “rancid” taste and odor of unsaturated oils which have been exposed to heat and oxygen for long periods of time—as in deep fryers. When an unsaturated fatty acid is hydrogenated, the double bond is broken and the fatty acid becomes saturated with hydrogen. Saturated fatty acids are resistant to rancidification. This quality is important to restaurants that serve foods prepared by deep frying. The taste and texture is better and the waste is less when saturated fats are used.
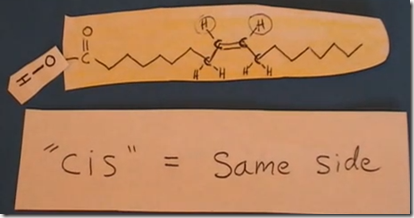
Rotation can not occur across a double bond so two configurations are possible. The cis isomer is the configuration found in vegetable oils and is shown here where both hydrogens are on the same side of the double bond.
The cis configuration bends the hydrocarbon chain on either side of the double bond as shown in this model.
This is the trans isomer of oleic acid, omega 9-trans-oleic acid. This trans-oleic acid can result from incomplete hydrogenation. Commercially, unsaturated oils are hydrogenated to improve shelf-life and prevent rancidification. However, trans fats are an undesirable result when hydrogenation is incomplete as in partially hydrogenated oils. Saturated fats and partially saturated fats improve the taste and texture of fried foods, but increases cholesterol associated with Low Density Lipoprotein (LDL or “bad” Cholesterol). It also decreases the High Density Lipoprotein (HDL or “good” cholesterol). Higher LDL cholesterol and lower HDL cholesterol is associated with coronary disease and atherosclerosis. This has generated controversy between the fast food industry and nutritional health groups.
Vegetable oils are composed of mostly unsaturated fatty acids and are liquid at room temperature. Vegetable oils may be hydrogenated and become solid at room temperature. These hydrogenated fatty acids from vegetable sources are are used in cooking as shortening and margarine.
Lard and butter are examples of saturated fats from animal sources. Saturated fatty acids in the diet are associated with higher levels of low density lipoproteins (LDL) in the bloodstream. The cholesterol associated with LDL is the cholesterol which is deposited in the walls of coronary arteries in the form of a plaque. These plaques can clog the arteries. They can also rupture which can cause the artery to clot off. This can lead to stroke (clotted artery to brain) or heart attack (clotted artery to heart).
Because of its association with plaque formation, LDL cholesterol is referred to as “bad cholesterol.” Because saturated fats and partially hydrogenated vegetable oils (trans fats) are are associated with higher LDL cholesterol levels they are considered less healthy than unsaturated fats.
Triglycerides are composed of 3 fatty acids joined to glycerol by ester bonds. Fatty acids are stored in biologic systems as triglycerides. In animals more of the fatty acids are saturated and this results in solid fat at room temperature. In plants more of the fatty acids are unsaturated and this results in most vegetable oils being liquid at room temperature.
Fats (triglycerides) are not soluble in aqueous solutions. However, when triglycerides are treated with a strong acid or base in water the ester bond can be broken to release glycerol (water soluble) and the free fatty acid.
The hydroxyl (OH) moity of the carboxylic acid molecule does not act like a hydroxide anion. The covalent bond between the oxygen and carbon is stronger than the covalent bond between oxygen and hydrogen in the hydroxyl group. Carboxylic acid is a proton donor, the hydrogen cation dissociates and carboxylic acid anions are produced. The carboxylic acid group can also participate in hydrogen bonds with proteins forming lipoproteins. In the blood stream, fatty acids are transported either as lipoproteins, free fatty acids or triglycerides.
Soap is made by treating fat with a strong base (potash = KOH) to convert the fat to water soluble glycerol and free fatty acid anions. This process is referred to as saponification. When soap and water are added to oil, the oil is dispersed into smaller particles (micelles) which can be washed away in the aqueous vehicle.
This reaction illustrated in this drawing is referred to as hydrolysis. The word hydrolysis refers to the fact that the ester bond is broken by adding a molecule of water. The reaction above may be summarized:
Triglyceride + H2O –> diglyceride + free fatty acid (FFA)
In order for our bodies to absorb fats, the triglycerides in our diet must be hydrolyzed into smaller particles. This reaction is catalyzed by lipase enzymes. While chewing, the fats are mechanically reduced in size and mixed with salivary or lingual lipase. In the stomach they are further reacted by gastric lipase. Finally in the small intestine the job is completed by pancreatic lipase. The anti-obesity drug orlistat, sold by the trade name Xenical®, works by inhibiting the pancreatic lipase enzyme. As a result, fats that are eaten can not be absorbed and therefore pass through undigested.
Organic compounds with hydroxyl (OH) functional groups are alcohols. The ‘ol’ ending is added when naming the alcohols. Glycerol is an alcohol. Glycerin is a common name for glycerol. The hydroxyl moiety in alcohols (OH covalently bound to carbon) have much different properties than the hydroxides in strong bases (OH– anion bound to metal cation). When an hydroxyl group is bound to a carbon which is bound to only one other carbon, it is a primary alcohol. If the carbon is bound to 2 other carbons, it is a secondary alcohol. Glycerol consists of 2 primary alcohols bound together by the secondary alcohol between them.
Triglycerides are formed in the body by combining a “glycerol backbone” with 3 fatty acid chains.
Transcription, notes and comments by James C. Gray, MD FACOG
Video by Janet Gray Coonce, MS
View the video then review the transcription, notes and comments:
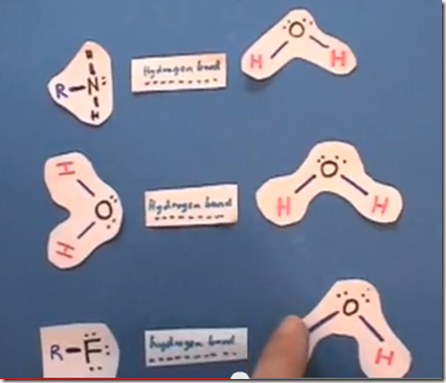
Hydrogen bonding is a special form of dipole to dipole interaction between molecules. Hydrogen bonding between molecules is possible when hydrogen is combined with nitrogen, oxygen or fluorine. Lets consider 2 molecules of water. We know that all molecules are attracted to each other by weak dispersion forces. Water molecules are also bipolar so we know they have dipole-dipole forces. Water has and even stronger force causing its molecules to be attracted to each other and that is hydrogen bonding. Hydrogen bonding is an intermolecular force in which a relatively positive charged hydrogen in one molecule is attracted to a relatively negative charged atom in an adjacent molecule.
In these Lewis structure diagrams of the water molecule, note that hydrogen is covalently bonded to oxygen. Oxygen has 2 pair of unshared electrons on one one pole and the two bonds to hydrogen are opposite. The molecules are dipolar with the hydrogen atoms positively charged relative to the negative forces of the oxygen on the adjacent molecule. In the case of water a strong hydrogen bond is formed which is the attraction between the oxygen of one molecule to the hydrogen of another molecule. This property of having strong hydrogen bonds gives water some of its unique properties. For instance, water is a liquid at room temperature whereas other molecules of similar weight and dipole configurations have much lower boiling points and are a gas at room temperature. Compare the boiling points in Fahrenheit of:
H2O = water = 212⁰ F
HF = hydrogen fluoride = 67⁰ F
NH3 = ammonia = -28⁰ F
The higher boiling point of H20 reflects the stronger hydrogen bonding between hydrogen and oxygen in water compared to the hydrogen bond in HF and NH3
In this illustration the notation δ+ |———> δ- (delta+ |———> delta– ) is used to indicate the partial charge difference across the covalent bonds in the water molecule. The arrow points in the direction of the more electronegative atom. Looking at the periodic table, electronegativity increases with atomic number within each period. In period 2, fluorine (F) is more electronegative than oxygen (O) which is more electronegative than nitrogen (N). They are all more electronegative than hydrogen (H) so the direction of δ+ |———> δ- in a hydrogen bond is always from hydrogen to the more electronegative atom. This indicates that there is a relative negative charge on the oxygen atom which is balanced by a relative positive charge split between the 2 hydrogen atoms.
Hydrogen covalently bonded to nitrogen, oxygen or fluorine may form hydrogen bonds with the nitrogen, oxygen or fluorine of another molecule. The hydrogen bond is not as strong as a covalent bond when electrons are shared. It is not as strong as an ionic bond when a full charge (electron) is transferred from the cation to the anion. It is a very strong dipole to dipole attraction. This special dipole-dipole attraction occurs when these 3 small electronegative atoms combine with hydrogen. The bond length is not as long. This “bond” is illustrated by using a dotted line as in this illustration.
Hydrogen bonding of water explains the physical states of water related to temperature. At a high temperature, water is a gas. When the temperature is above the boiling point, the water molecules are moving very rapidly and have too much energy to be attracted to each other by hydrogen bonds so they disperse separately as a gas. As the temperature cools, the water molecules do not move as rapidly and are attracted to each other causing water to condense into a liquid form. As a liquid, it can be confined to a smaller container than a gas at the same pressure. If the temperature falls below the melting point, the water molecules slow down and the hydrogen bonds begin to form a lattice which we know as snow flakes or ice crystals.
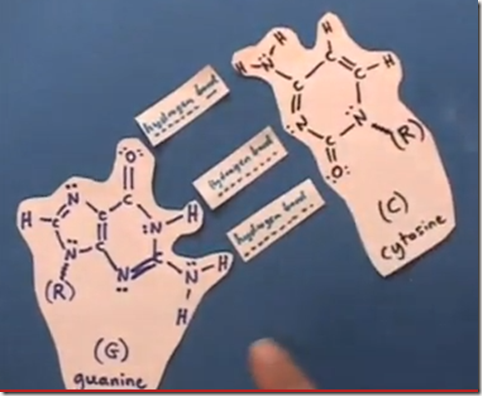
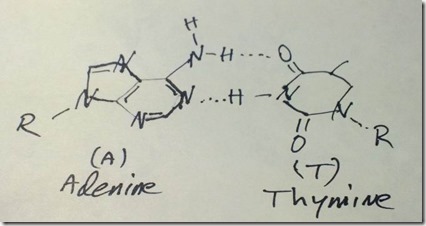
Hydrogen bonds are also important in biochemistry and vital to life processes. They hold proteins and enzymes together. They also hold DNA together.
This shows how hydrogen bonds link the base pairs guanine and cytosine together in the DNA helix as proposed by Watson and Crick in 1953. The “R” group is the sugar-phosphate backbone of the helix.
Adenine forms hydrogen bonds with thymine as the other base pair in DNA.
Transcription, notes and comments by James C. Gray, MD FACOG
Video by Janet Gray Coonce, MS
Review transcription, notes and comments after reviewing video:
Now I would like to go through several polyatomic anions and cations, their name and atomic structure. An ion is an atom or molecule in which the total number of electrons do not equal the total number of protons. Since the number of electrons and protons are not equal, the atom or molecule has either a positive or negative charge. An anion is an ion with more electrons than protons. It has gained at least one extra electron so it has a negative charge. Anions (negative ions) migrate toward the anode or positive electrode in a direct electric current. A cation is an ion with more protons than electrons. It has lost at least one electron from its neutral state so it has a positive charge. Cations (positive ions) migrate toward the cathode or negative electrode in a direct electric current. A polyatomic anion is a negatively charged ion composed of more than one atom. The atoms which make up a polyatomic anion share their electrons in covalent bonds. The atoms which are covalently bonded stay together when the ion forms an ionic bond to an ion with opposite charge.
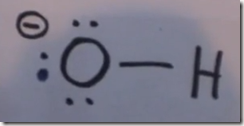
Consider the hydroxide anion:
OH– hydroxide anion
Hydroxide ion is a very strong base. A base is a proton (hydrogen ion) acceptor. An acid is a proton (hydrogen ion) donor. If the hydroxide ion can combine with a proton (hydrogen ion) from an acid it will react to form water. The oxygen and hydrogen share a covalent bond so they stay bonded together. Overall the hydroxide ion has a negative charge so there must be an associated cation to balance the charge. There are many examples of common cations which form ionic bonds with OH–. Frequently encountered examples include Na+, K+, NH4+, Ca++, Mg++, and Al+++, but there are many more. Whenever the hydroxyl ion bonds with these cations, the oxygen and hydrogen stay together and the overall charge is –1. The number of OH- anions which bind with the cation will depend on the charge of the cation. When writing the symbol, parentheses are used to define the anion and a subscript indicates the number of anions bonded to the cation.
Examples: NaOH, Ca(OH)2, Al(OH)3.
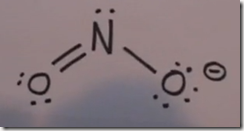
NO3– nitrate anion
The overall charge of the nitrate anion is –1. Nitrogen is in group VA of the periodic table and has 5 valence electrons. Like oxygen, nitrogen is in period 2 and it cannot expand its octet. This explains why there is only a single bond between the nitrogen and 2 of the oxygen atoms. This results in the separation of charge with nitrogen having a positive charge and 2 of the oxygen atoms having a negative charge. The net result is a negative 1 charge for the nitrate anion. Compare this Lewis dot structure with the nitrite anion.
The nitrogen and 3 oxygen atoms are covalently bonded. The 4 atoms stay together when they bond with the associated cation. Nitrate could also form an ionic bond with the same cations as we used in the example for hydroxide. The charges must balance. The nitrogen and 3 oxygen atoms will stay together and overall the nitrate charge is –1..
Examples: NaNO3, Ca(NO3)2, Al(NO3)3.
========================
Cations have a positive charge because the cation has lost an electron to the associated anion.
NH4+ ammonium cation
Note that the nitrogen atom is only sharing 4 electrons in covalent bonds with hydrogen. Nitrogen is in group VA of the periodic table and has 5 valence electrons in its natural state. In the ammonium ion, nitrogen has lost one of its electrons and therefore has a positive charge of +1.
H3O+ hydronium cation
In this Lewis structure of the hydronium ion, oxygen is sharing 3 of its valence electrons in covalent bonds with hydrogen and there is a single pair of unshared electrons. 3 + 2 = 5. Oxygen is in periodic table group VIA and has 6 valence electrons in its natural state. Therefore in the hydronium ion, oxygen has lost one of its electrons(reduced from 6 to 5). This gives the overall hydronium ion a +1 formal charge. However if you count all the electrons the oxygen atom “feels like it has”, shared and unshared, in oxygen’s valence shell, its octet is full. This is because one of the hydrogen atoms is sharing an electron which was a member of a lone pair in the natural state. The electron which was paired in the natural state was “lost” to the associated anion. The point is the hydronium ion is a stable cation because the oxygen’s octet is full. Each hydrogen has its duet of electrons, but the ion has gained a proton and therefore has a net positive charge.
NO2– nitrite anion
H2PO4– dihydrogen phosphate anion
Again, one of the oxygens have an extra electron giving the anion its negative charge.
MnO4– permanganate anion
Oxygen has gained an extra electron and gives the anion its negative charge.
HSO4– hydrogen (bi)sulfate anion
Here the net negative charge is due to an extra electron on the oxygen on the right.
HCO3– hydrogen (bi)carbonate anion
A commonly used bicarbonate is sodium bicarbonate or baking soda in which case sodium is the associated cation. In this Lewis structure of the bicarbonate anion, the oxygen on the left has picked up an additional electron, giving it a full octet but with a negative charge. Bicarbonate ion is a buffer. The oxygen with the extra electron can accept a proton (hydrogen cation) and act as a base, or it can give up a proton from the oxygen atom on the right in which case it would be acting as an acid.
CN– cyanide anion
Both carbon and nitrogen have a full octet, however, carbon has picked up an extra electron from the associated cation.
CH3COO– (or C2H3O2– or OAc– ) acetate anion
Note the formal negative charge on this structure. This is due to an extra electron associated with oxygen which was picked up from the associated cation. Count the electrons. The oxygen with the negative charge has 7 electrons. Oxygen is in group VIA so it only has 6 electrons in the elemental natural neutral state. This extra electron which is responsible for the negative charge was donated by the associated cation.
Now lets go over some anions with a negative 2 charge.
CO32- carbonate anion
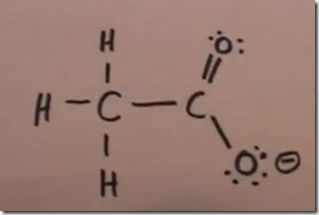
C2O42- oxylate anion
CrO42- chromate anion
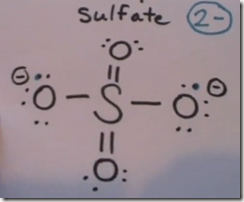
SO42- sulfate anion
HPO42- monohydrogen phosphate anion
Look at the different distribution of valence electrons around the oxygen atoms in HPO42–. One of the oxygen atoms has a double bond to phosphorus and 2 lone pairs. Another oxygen has a single bond to hydrogen and another single bond to phosphorus with 2 lone pairs. Two of the atoms have 3 lone pairs and a single bond to phosphorus. These two atoms each as a charge of 1- and are responsible for the overall charge of the phosphorus anion of 2-.
PO43- phosphate anion
Problem: Draw the structure for dihydrogen phosphate:
H2PO4– dihydrogen phosphate anion
Transcription, notes and comments by James C. Gray, MD FACOG
Video by Janet Gray Coonce, MS
The term chiral describes a molecule whose arrangement of atoms is such that it cannot be superimposed on its mirror image. If the molecular structure of a substance is chiral, it shows handedness.
In this illustration, the right-hand glove is a mirror image of the left-hand glove.
In chemistry, chemical structures which are composed of the same atoms but structurally are different form their mirror images are chiral structures. They have the same atoms in the same ratios, but their structures are different. This means that the molecule will show handedness like these gloves. The left handed glove will fit the left hand but not the right hand. The right handed glove will fit the right hand but not the left hand. They are mirror images of each other but they are not identical. They are chiral. They can not be superposed onto each other.
This characteristic of chemical compounds is very important in biochemistry and pharmacology. Chemical reactions in biologic systems are catalyzed by large molecules referred to as enzymes. These enzymes may be activated or inactivated by bonding to certain molecules. The drug industry takes advantage of this by manufacturing drugs which may bind to these enzymes for the specific purpose of either activating or inhibiting the action of the enzyme. Other drugs are molecules which bind to locations on certain cells referred to as receptor sites. These receptors control specific biochemical functions in the cell. Many drugs rely on the ability to either turn on (receptor agonist) or to turn off (receptor antagonist) cellular activity wich is governed by the particular receptor site.
For the enzyme or receptor site to be activated by a molecule the molecule must “fit” on the enzyme or receptor. This is why chirality is important.
In the example imagine the receptor in the shape of a left-handed glove. The left hand can fit the glove perfectly and turn on the receptor. However the right-handed glove cannot fit and cannot turn on the receptor.
Here a mirror image is illustrated of a tetrahedral carbon compound with 4 bonds to different atoms.
These are chiral compounds. They have the same formula. They differ only in the three-dimensional orientations of the atoms in space which are bound to the central carbon atom. They are mirror images but there is no plane of symmetry. The structures cannot be superimposed on each other. In this image the green and white are superimposed, but the red and blue are opposite. The structures have the same molecular formula but cannot be superimposed on each other. There is no plane of symmetry. They are chiral compounds.
Whys is this important?
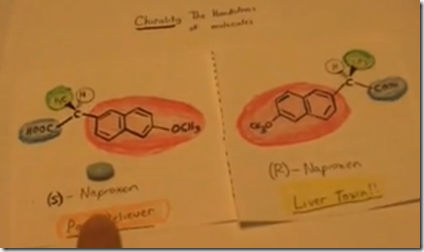
Here is the molecular structure for (S)- naproxen. It is common over-the-counter pain reliever. (S)-naproxen binds to the enzyme cyclooxygenase and inhibits its action in the synthesis of prostaglandins. Decreasing prostaglandin production at the site of injury decreases pain and inflammation. Originally the drug was available as prescription as the brand name Naprosyn. It is now over-the-counter as Aleve and many other generic brands.
Only the (S)-naproxen isomer will bind to and inhibit the cyclooxygenase enzyme to decrease prostaglandin production. (R)-Naproxen is the mirror image of (S) Naproxen. Although it has the same chemical formula, it’s structure is different. It does not inhibit the cyclooxygenase enzyme and it is toxic to the liver.
Here the (R) form is drawn adjacent to the (S) form. Both forms have the same molecular formula, C14H14O3 but are different structurally and this results in different biological effects. The structural difference is due to the different arrangement of groups (H-, H3C-, HOOC-)-surrounding the central carbon stereo-center. (R)- and (S)- naproxen are enantiomers: stereo-isomers are mirror images which are non-superimposable. When a central carbon atom is bound to 4 different groups, switching any two groups will result in another enantiomer.
A polarimeter is device which measures the angle of rotation of polarized light which passes through a substance. A polarimeter may be used to determine which enantiomer is present by the direction in which polarized light is rotated when passed through a solution of the substance. The light may be bent counter clockwise (L) or clockwise (R).
A very similar compound also used as a pain reliever is Ibuprofen. It is sold as a racemic mixture containing both the (S)- and the (R) enantiomers. (S)-ibuprofen is the active drug and the (R)-ibuprofen is just not active.
Pharmacology Example: Another real world example of this is the drug norgestrel. Norgestrel is a chiral compound. There are two structures possible and they are mirror images of each other. The symbol for norgestrel is C21H28O2. The structural representation of the left-handed active component is:
This “left-handed” isomer of norgestrel is named after its handedness, levonorgestrel or L-norgestrel is named “L” because polarized light passed through a solution containing this form will bend the light counter clockwise (Left). It happens to fit perfectly on and will activate progesterone receptors which are concentrated in target tissues related to reproduction in women. Specifically progesterone receptors are located in the pituitary gland, ovary, uterus, and breast. Normally when progesterone activates these receptors, it signals that an ovum has been released. If the egg is fertilized progesterone will be produced by the placenta until the baby is delivered. When the progesterone receptors are activated, the chemical machinery in the target organs change from stimulating the release of or receiving another ovum, to reactions necessary to support pregnancy and prevent the release of another ovum. This is how the birth control pill works. When norgestrel was originally manufactured, a racemic mixture containing both the left and right-handed components were in the patented drug (Ovral). The right-handed component (dextronorgestrel or D-norgestrel) is harmless but inactive. When the manufacturer discovered how to manufacture the pure left-handed L-norgestrel, they applied for and were awarded another patent for Nordette which contained only the active L-norgestrel. This occurred just as their patent on Ovral (racemic norgestrel) expired. The drug manufacturer very cleverly capitalized on chirality.
The “right-handed” norgestrel, D-norgestrel has no medical use but could be drawn exactly as above but all structures coming at you at the carbon stereocenters would be going away and vice versa. In other words, the dotted lines would be replaced by wedges, and the wedges by dotted lines.
Our bodies recognize the differences in chiral compounds
Chiral compounds “show handedness.”
Compounds which do not show handedness are “achiral.”
A chiral carbon is a stereocenter carbon. It is a carbon with 4 different groups attached.
Enantiomers are stereoisomers with non-superimposable mirror images.
A stereocenter or stereogenic center is an atom bearing groups that an interchanging of any two groups leads to a stereoisomer.
Transcription by James C. Gray, MD FACOG
Video by Janet Gray Coonce, MS
Continuing to solve the problem discussed at the end of Part II…..
Problem: Use the Born-Haber Cycle to calculate the lattice energy of MgF2
The known enthalpies of each of the steps of the Born-Haber cycle are obtained from a table:
- Enthalpy of Sublimation for Mg = ΔHsub = + 146 kJ/mol
- 1st ionization energy for Mg = IE1 = +738 kJ/mol
- 2nd ionization energy for Mg = IE2 = +1451 kJ/mol
- Bond dissociation Energy for F2 (g) = BE = +159 kJ/mol
- Electron Affinity of F (g) = EA = –328 k/J/mol
- Enthalpy of formation of MgF2 (s) = ΔHof = –1124 kJ/mol
Now, writing the component reactions of the Born-Haber Cycle:
Mg(s) –> Mg(g) ΔHsub = + 146 kJ/mol
Mg(g) –> Mg+(g) + 1 e– IE1 = +738 kJ/mol
Mg+(g) –> Mg+2(g) + 1 e– IE2 = +1451 kJ/mol
F2(g) –> 2F(g) BE = +159 kJ/mol
F(g) + 1 e– –> F–(g) EA = –328 k/J/mol
Mg(s) + F2(g) –> MgF2(s) ΔHof = –1124 kJ/mol
Hess’s law states that the total enthalpy change during the complete course of a reaction is same whether the reaction is made in one step or in several steps. Remember the lattice energy is energy released when ions in the gaseous form combine to form a solid. For the case of MgF2(s):
Mg2+(g) + 2F-(g) –> MgF2(s) ΔH = Lattice Energy
To calculate the total enthalpy change using Hess’s law we will need to find the enthalpy change for each component of the Born-Haber cycle and add them together. Remember if we reverse the direction of the reaction, the sign of the change in enthalpy will change. So to write the enthalpy reactions required in the formation of MgF2(s), we need to write the reactions in the direction required and we need to adjust for the number of moles of each reactant used:
- 2F–(g) – –> 2F(g) + 2 e– 2EA = 2(+328 k/J/mol) = 656 kJ/mol
- Mg+2(g) + 1 e– –> Mg+(g) IE2 = -1451 kJ/mol
- 2F(g) –> F2(g) BE = –159 kJ/mol
- Mg+(g) + 1 e– –> Mg(g) IE1 = +738 kJ/mol
- Mg(g) –> Mg(s) ΔHsub = – 146 kJ/mol
- Mg(s) + F2(g) –> MgF2(s) ΔHof = –1124 kJ/mol
Now, according to Hess’s Law all we need to do is to add all the enthalpies associated with each step of the Born-Haber Cycle to calculate the lattice energy.
| 1. | -1124 |
| 2. | + 656 |
| 3. | -1451 |
| 4. | – 159 |
| 5 | – 738 |
| 6 | – 146 |
| Total | -2862 |
Therefore lattice energy of MgF2 ΔH= -2862 kJ/mol
Transcription by James C. Gray, MD FACOG
Video by Janet Coonce
Be sure to watch video’s Lattice Energy Part I and Part II before reviewing the transcription. Part II begins with a continuation of the discussion on EA: Electron Affinity.
Chlorine is happier (more stable) as a charged gaseous ion (g) than a gaseous free radical (g) with an unshared electron. Chlorine needs an electron to complete its octet. This increased stability is confirmed by the change in enthalpy. The electron affinity for chlorine can be found on a table of known electron affinities and is expressed as the delta H:
Δ H = – 349 kJ/mol
Since heat is released, the reaction is exothermic and the Δ H is a negative number. That means that the chloride anion, Cl- (g) is more stable than the chlorine free radical, Cl (g). A free radical is an atom with at least one lone electron in its outer shell. Remember that Cl normally exists as a diatomic molecule, Cl2 gas. We discussed the energy required to break the Cl—Cl bond in the discussion of dissociation energy.
The next term you will need to understand to calculate lattice energy using the Born-Haber Cycle is Lattice Energy itself.
5. Lattice Energy is the enthalpy change (Δ H) that accompanies the formation of 1 mole of an ionic solid from its separated gaseous ions.
In the case of reacting gaseous sodium cation and gaseous chloride anion to form a lattice of sodium chloride solid, the reaction is expressed symbolically as:
Na+ (g) + Cl- (g) ——> NaCl(s)
By looking at a table of known values we determine the change in enthalpy or lattice energy to be negative 787 kJ/mol. The number is negative and energy is lost as heat. The reaction is exothermic. Symbolically the lattice energy is expressed as the change in enthalpy associated with forming the solid lattice:
Δ H = – 787 kJ/mol Negative means heat was lost (exothermic) and the reaction resulted in a lower energy state
To convert the NaCl solid back to the gaseous ion state, enough heat would have to be added to break the lattice bond. To indicate that heat is added, the lattice energy would be expressed as a positive 787 kJ/mol:
NaCl(s) —-> Na+ (g) + Cl- (g)
Δ H = + 787 kJ/mol Positive means heat was used (endothermic) to move to a higher energy state
In your text book if lattice energy is defined as the amount of energy required to heat a solid to the gaseous ions, the lattice energy will be a positive number. If it is defined as the energy released when the gaseous ions combine to form the solid lattice, then the lattice energy will be a negative number. The absolute value is the same. The sign reflects the direction of the reaction depending on whether energy (heat) was added or removed.
The final term you will need to know to calculate lattice energy using the Born-Haber Cycle is the standard enthalpy of formation.
6. Standard Enthalpy of Formation:
This is another value that is available in a table of known values. The symbol to indicate the standard enthalpy of formation is:
ΔHof
The superscript ‘’o’’ indicates a ‘’standard’’ given temperature and 1 atomosphere pressure. The subscript ‘’f’’ indicates that this is the formation of 1 mole of a substance from the reference form of an element in its standard state.
Sodium is a metal in group I of the periodic table. In its standard state it is a solid. Chlorine in its standard state is a diatomic molecule. Since we want to form only 1 mole of NaCl we only need 1/2 mole of Cl2 (g) which explains the use of a fraction to balance the equation. We look at a table of known values and find that the standard enthalpy of formation of NaCl is expressed as:
ΔHof = –411 kJ/mol
So how do we use the Born-Haber Cycle to calculate the lattice energy of an ionic compound? Let’s work a typical problem you might see in a textbook.
Problem: Use the Born-Haber Cycle to calculate the lattice energy of MgF2
Given:
- Enthalpy of Sublimation for Mg = ΔHsub = + 146 kJ/mol
- 1st ionization energy for Mg = IE1 = +738 kJ/mol
- 2nd ionization energy for Mg = IE2 = +1451 kJ/mol
- Bond dissociation Energy for F2 (g) = BE = +159 kJ/mol
- Electron Affinity of F (g) = EA = –328 k/J/mol
- Enthalpy of formation of MgF2 (s) = ΔHof = –1124 kJ/mol
Now, writing the component reactions of the Born-Haber Cycle:
Mg(s) –> Mg(g) ΔHsub = + 146 kJ/mol
Mg(g) –> Mg+(g) IE1 = +738 kJ/mol
Mg+(g) –> Mg+2(g) + 1 e- IE2 = +1451 kJ/mol
F2(g) –> 2F(g) BE = +159 kJ/mol
F(g) + 1 e- –> F-(g) EA = –328 k/J/mol
Mg(s) + F2(g) –> MgF2(s) ΔHof = –1124 kJ/mol
This transcription will continue in Lattice Energy Part III
Transcription by James C. Gray MD FACOG
Video by Janet Gray Coonce, MS
In this video I will discuss lattice energy and how to use the Born-Haber cycle to calculate lattice energy from known enthalpies of certain reactions.
What is lattice energy? Lattice energy is the energy released when ions in the gaseous form are combined to form a solid ionic compound.
Na+ (g) + Cl– (g) —-> NaCl(s)
ΔH = –787 kJ/mol
In this symbolic expression, gaseous sodium ion and gaseous chloride ions combine to form a more stable solid sodium chloride. Whenever a reaction goes to a more stable compound, heat is released. The magnitude of the energy released is the lattice engergy expressed as a ΔH of NEGATIVE 787 kJ/mol. Since energy was lost, the ΔH is a negative number. This change in thermal energy, or change in enthalpy associated with the reaction is represented by the symbol ΔH. If the reaction were to be reversed, it would require adding heat to break the solid up into gaseous ions. That reaction would require a ΔH of POSITIVE 787 kJ/mol. So to summarize, if heat is lost, the change in enthalpy or ΔH is negative. Since heat is given off, it is an exothermic reaction. If heat is added, the change in enthalpy or ΔH is positive. When heat is used by the reaction, the reaction is an endothermic reaction. Lattice energy is released when gaseous ions come together to bond as a solid. It is an exothermic reaction. The change in enthalpy or ΔH is negative. The solid form must be heated in order to convert the solid back to the ionic gaseous form. Since heat is added, it is an endothermic reaction and the change in enthalpy or ΔH is positive.
How does the size of the anions and cations affect the magnitude of lattice energy? Smaller atoms are closer together and are held more strongly than larger atoms. Therefore the magnitude of the change in enthalpy is higher for the smaller molecules.
How does the size of the cation and anion affect the magnitude of the lattice energy? The smaller the atoms, the stronger the attraction (ionic bond) between them. So looking at the table, the absolute value of lattice energy is predictably larger for LiF (-1036) than for NaCl (-787) or at the bottom RbI (-630). The value of the lattice energy is negative or positive depending upon whether the ions are coming together as a solid compound and therefore negative or whether they are split into the gaseous ions in which case the value would be positive. The absolute value of the numeric magnitude is the same. Again, energy is required (positive lattice energy) to break a bond. Energy is released (negative lattice energy) when a bond is formed.
Repeating the definition: Lattice Energy is the energy released when gaseous ions combine to form a solid ionic compound.
We have discussed the inverse relationship between the size of the ions and the magnitude of lattice energy released when the ions form a bond. The charge also affects the lattice energy.
The higher the absolute value of the charge of the ion, the stronger the ionic bond. For example, the absolute value of the lattice energy released by the reaction of Al3+ + 3OH– ( | -5627|= 5627) is higher than when Na+ + OH– ( | -900|= 900) are combined. This is because the higher charge of the Al3+ cation when compared to the Na+ cation. The same is true for the charge of the anion. Look at the chart and compare the lattice energy of Na+ + OH– ( | -900|= 900) with Na+ + O2- ( | -2481|= 2481). The absolute value of the lattice energy released is larger associated with Na+ +O2- than with Na+ + OH– due the more negative charge of the O2– anion.
To restate this concept in other terms, the higher the absolute value of the valence of the ion ( |valence| ), the higher the lattice energy of the ionic compound. This means the compound is much more stable. It will take more energy to break the bond. The formation of the solid ionic compound from the gas is exothermic and more heat will be given off when compared to formation of compounds with ions of lower valence. Look at the chart and note the lattice energy when Al3+ + O2– are combined. This is a very exothermic reaction and the absolute value of lattice energy released is |-15916|. Since energy is released from the system it is expressed as a negative number. If heat were applied to the solid compound, 15916 kJ/mol would have to be added to break the ions back into their gaseous state. When energy is added, the reaction is endothermic and the lattice energy is expressed as a positive number. The absolute value (magnitude of energy change) is the same.
This diagram illustrates the change in lattice energy when gaseous form of the lithium cation and fluorine anion are combined to form lithium fluoride. The gaseous ions are at a higher energy state than the lithium fluoride solid compound. This difference in energy state results in heat being released with the formation of the solid compound. Since energy is release, it is an exothermic reaction. The magnitude of this change is the lattice energy.
To write this reaction symbolically:
Li+ (g) + F- (g) —————–> LiF (s) + 1036kJ/mol heat released
Looking at the known enthalpy chart, the lattice energy ( ΔH ) = –1036 kJ/mol
If the direction of the reaction were changed from solid to gaseous, then the reaction could be expressed:
Li+ (g) + F- (g) <—————– LiF (s) + 1036kJ/mol heat or
LiF (s) + 1036kJ/mol heat ————————> Li+ (g) + F- (g)
ΔH = 1036 kJ/mol
Sometimes its not so easy to directly calculate the lattice energy taking the gaseous ions that form that solid, so what we do is indirectly calculate the lattice energy using the Born-Haber cycle.
To use the Born-Haber Cycle, you have to understand a few terms.
1. Enthalpy of Sublimation:
Sublimation is when a solid is converted to a gas. When particles are released in the gaseous form from a solid substance the change in enthalpy or ΔH is going to be positive because energy will have to be added to break those bonds. In the case of elemental sodium illustrated above, the enthalpy of sublimation is 107 kJ/mol. That means that 107 kJ/mol of heat will have to be applied to the sodium metal to release the sodium as a gas. The change in enthalpy (heat required) is expressed symbolically as:
Na (s) + heat ———> Na (g)
ΔH = +107 kJ/mol
Values of the enthalpy of sublimation for known substances may be found in tables where the values have been calculated by physical chemists. For this lesson you need to know that these are calculated values, but we will look them up in a table. We will leave the “how to calculate” the values up to the physical chemists.
Another term you need to understand to use the Born-Haber Cycle to calculate lattice energy is:
2. Bond Dissociation Energy
Bond dissociation energy is the energy required to dissociate or break a bond between atoms. In the illustration above, using any of the halogens designated by the symbol X, there is a homolytic cleavage of the bond between X—X (gas) to release 2 atomic radicals of the halogen atom.
The bond dissociation energy to break a molecule of chlorine gas into 2 atoms of chlorine radicals can be expressed by the equation given in the example at the bottom of the diagram.
The same reaction can be expressed as a Lewis dot structure to illustrate what happens to the valence electrons. The bond dissociation energy for this reaction is expressed as:
ΔH = +243 kJ/mol The positive number indicates that energy is required before diatomic chlorine gas will dissociate into free radicals.
The 3rd term you will need to understand in order to use the Born-Haber Cycle to calculate lattice energy is the Ionization Energy, IE.
3. IE: Ionization Energy
The ionization energy is the energy required to remove an electron from a gaseous ion or atom. In this illustration the ionization energy required to convert Na gas to a Na+ ion by the removal of an electron is expressed by:
ΔH = +496 kJ/mol This is the amount of heat which would be required for this step.
The smaller the atom, the lower the amount of energy required to release an electron. More energy is required by atoms to the right of Na in the periodic table. More energy is also required for atoms below Na in the periodic table. Since Na is in group 1 and period 3, it is a relatively small atom and will have a lower ionization energy than elements in the other groups in period 3. It will also have a lower ionization energy than elements in periods below period 3.
Na is an alkali metal, a member of group I in the periodic table, and has only one electron in its outer shell which can be removed. What would we have to consider instead of Na, we chose Mg, an alkaline earth metal, a member of group II in the periodic table? Members of the alkaline earth metals have 2 electrons in their outer shell which can be removed. For these elements there are 2 ionization energies.
The ionization energy for the first electron, and the ionization energy to release the second electron. It will require more energy to remove the second electron than the first so the second ionization energy will be higher than the first ionization energy. So the first ionization energy is the amount of energy required to remove one electron from the outermost shell of an atom in its gaseous state.
The last consideration of terms you need to know before you can calculate lattice energy using the Born-Haber Cycle is Electron Affinity, EA.
4. EA: Electron Affinity
Electron affinity is the energy released when an electron is added to a neutral atom in its gaseous state to form an ion.
In the case of chlorine gas radicals (produced by dissociating the bonds of diatomic chlorine gas discussed above), adding an electron releases 349 kJ/mol. The fact that energy is release means that chlorine ion gas is more stable than the chlorine gas radical and the change in enthalpy is a negative number.
ΔH = -349 kJ/mol
Transcribed by James C. Gray MD FACOG