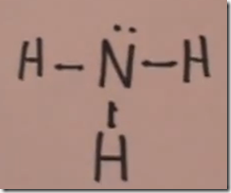Lewis Dot Structures (2): Water and Ammonia
Video by Janet Gray Coonce MS
If you plan to view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges.
Review the notes after watching the video:
How to draw the Lewis dot structure for H20? We know that it would have to be H-O-H because hydrogen can only form one bond. Hydrogen is always on the outside of a molecule. Therefore oxygen would have to be in the middle.
To draw the Lewis structure, we first put dots in for the VALENCE electrons. Valence electrons are the electrons available in the outer shell of an atom. Hydrogen has only 1 valence electron and gets 1 dot each representing the lone electron associated with the hydrogen proton. Oxygen has 6 valence electrons. A full outer shell for oxygen would have 8 valence electrons, 4 groups of 2. Therefore we draw 2 paired electrons and 2 unpaired electrons. To form a stable compound, hydrogen would like to have 2 more electrons in it’s outer shell to complete the the octet of 8 electrons.
Both hydrogen and oxygen will be able to have their VALENCE shell of electrons filled if they each SHARE their unpaired electrons with the other. This way they each will have a pair of electrons in a COVALENT BOND between them. In the top illustration a line was drawn between the unpaired electron dots. This was re-drawn for neatness sake in the bottom drawing. By bonding covalently with another atom, each atom has a full valence shell of electrons and remain electron neutral.
Lewis Structure of ammonia gas, formula NH3
Ammonia solution used as a cleaning solution is ammonia gas NH3 which is dissolved in water. This can be expressed by the equation NH3 +H20 –> NH4+ +OH–. You are familiar with the pungent odor of ammonia gas which is also used as a respiratory stimulant (smelling salts).
In this case nitrogen is the central atom. Nitrogen is in group V-A of the periodic table. It has a total of 5 valence electrons with 3 unpaired electrons which are available for covalent bonding. Hydrogen is in group IA and has one unpaired electron available for covalent bonding.
Here a line is drawn between the dots representing the electrons involved in each of the covalent bonds. When these electrons are covalently shared, nitrogen has a total of 8 valence electrons and each hydrogen has 2 valence electrons. The Lewis dot drawing makes it clear that nitrogen has completed its octet with 3 covalent bonds with hydrogen and 1 lone pair. Nitrogen now with its full octet of valence electrons is isoelectric with the noble gas neon. Hydrogen with 2 electrons in its outer shell is isoelectric with the noble gas helium.
–Transcription by James C. Gray MD FACOG