Electronic & Molecular Geometry
Videos by Janet Gray Coonce, MS
If you plan to view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges.
In this tutorial I want to help you visualize the electronic and molecular geometries predicted by the valence-shell electron-pair repulsion, VSEPR, models. I will demonstrate how to represent 3 dimensional models of electronic and molecular geometry in 2 dimensional drawings.
There are 3 regions of electron density in the acetylene (C2H2) molecule. Each carbon is flanked on the outside by a single bond to hydrogen. The two central carbon atoms are sharing a triple bond. So each of the carbon atoms have 2 electron dense areas trying to get as far apart as possible. When 2 electron dense areas connected to a central atom get as far apart as possible, they will align themselves across a straight line. Acetylene therefore has linear molecular geometry. The bond angle of a straight line is 180 degrees.
Carbon dioxide, C02 is another example of a central atom with two areas of electron density repelling each other. They oxygen atoms are getting as far apart from each other as possible and form a straight line. It is linear electronic and linear molecular geometry.
If there are 3 identical atoms connected to a central atom, each will repel each other and get as far apart as possible. They will assume a trigonal planar electronic and trigonal planar molecular geometry. All 3 of the atoms bound to the central atom will be in the same plane and will be equal distant. In the case of 3 the bond angle will be 120 degrees (360/3).
Here is a central atom with 3 electron dense areas but only a lone pair of electrons is in one of the 3 areas. The lone electrons will be more electronegative than the atoms sharing electrons in a bond. The bonded atoms will be repelled by the force of the lone pair (blue arrows) and the bond angle will be less than 120 degrees. The molecular shape will be bent. The electronic geometry is still trigonal planar.
When there are 4 atoms bonding to a central atom and 4 regions of electron density, the electronic and molecular shape is tetrahedral. The atoms will arrange themselves equally around the central atom in 3 dimensions. In the 2 dimensional representation, the solid wedge represents an atom projecting up from the plane of the paper. The dotted wedge represents an atom projecting below the plane of the paper as illustrated.
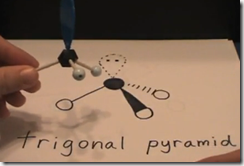
If one of the 4 areas of electron density was a lone pair, the molecular shape would be a trigonal pyramid (3 atoms attached to central atom). The electronic shape would still be tetrahedral (4 areas of electronic density around the central atom).
If 2 of the 4 areas of electron density is represented by a lone pair of electrons and 2 electrons shared in a bond with another atom, then the less electronegative atoms in the bond will be repelled by the more electronegative regions occupied by the lone electrons. The electronic shape would still be tetrahedral but the molecular shape would be bent.
If a central atom shares 5 electrons by forming covalent bonds with 5 other atoms the electronic and molecular geometry is a trigonal bipyramid.
If one of the 5 areas of electron density is occupied by a lone pair of electrons, the electronic shape would still be trigonal bipyramid but the molecular shape would be a “see-saw.”
If 2 areas are occupied by lone pairs the molecular geometry will be T-shaped.
If there are 3 pairs of unshared electrons and 2 bonds, the molecular geometry will be linear. The electronic geometry is still trigonal bipyramid. This is the shape when 5 regions surrounding a central atom try to repel each other in order to get as far apart as possible.
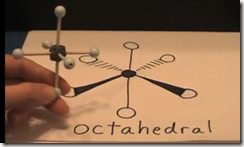
With 6 regions all repelling each other to get as far apart as possible, the molecular and electronic geometry is octahedral.
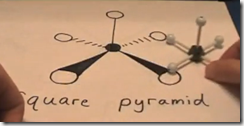
If one of the 6 regions is a lone pair, the molecular geometry would be a square pyramid. The electronic geometry would still be an octahedral.
If 2 of the regions are occupied by a lone pair of electrons then the molecular geometry would be square planar. There are still 6 regions of electron density so the electronic geometry is still octahedral.
Review: Regions of electron density (RHED) surrounding a central atom are of 4 types:
1. single bond = a RHED consisting of 1 pair of shared electrons
2. double bond = a RHED consisting of 2 pairs of shared electrons
3. triple bond = a RHED consisting of 3 pairs of shared electrons
4. a RHED consisting of lone pair of unshared electrons.
Here is the Lewis structure for carbon dioxide, CO2. How many electron regions around the central carbon atom? Answer: There is a double bond on each side. A double bond is a single region. Therefore we know 1 + 1 = 2. So the answer is there are 2 regions. What about the molecular shape? If the electron regions associated with the more electronegative oxygen atoms repel each other and try to get as far apart as possible, what molecular shape will the CO2 molecule have? Linear. A straight line. A bond angle of 180 degrees. What is the geometry of the electron regions? They are also in a straight line. Linear.
Summary: CO2 has 2 electron regions (RHED) around the central carbon atom with no lone pairs of unshared electrons.
Electronic geometry: Linear
Molecular geometry: Linear
Here is the Lewis structure for ammonia gas, NH3. How many regions of high electron density (RHED) are there around the central nitrogen atom? Count them. There are 3 single bonds and a single lone pair of electrons. 3 + 1 = 4. If all of these electronic regions get as far apart as possible what will be the 3 dimensional shape? There are 4 points and 4 points in 3 dimensions is a tetrahedral. Therefore the electronic shape is tetrahedral. However, there are only 3 atoms around the central nitrogen. What is the MOLECULAR geometry? The 3 hydrogen atoms form a triangle in one plane and the nitrogen is above it. It is a trigonal pyramid.
Summary: Ammonia gas, NH3 has 4 electron regions (RHED) around the central nitrogen atom with 1 pair of unshared electrons.
Electronic geometry: Tetrahedral
Molecular geometry: Trigonal pyramid
Here is the Lewis structure for water, H20.
There are 4 RHED, 2 lone pairs and 2 single bonds. The 4 electronic regions will get as far apart as possible in a tetrahedral electronic geometry.
There are only 2 atoms attached to the central oxygen atom and they will be repelled by the lone pairs. Although there are only 2 atoms bonded to oxygen and they are trying to get as far apart from each other as possible, the three atoms are not in a straight line. The hydrogen atoms are also being repelled by the lone pairs of electrons. The molecular geometry is not a straight line. The molecular geometry is bent.
Summary: Water, H20, has 4 electron regions around the central oxygen atom with 2 pair of unshared electrons and 2 single bonds.
Electronic geometry: Tetrahedral
Molecular geometry: Bent
Summary of molecular geometries for each of the electronic geometries:
A. Trigonal Planar Electronic geometry = 3 RHED
1. 3 bonds + 0 lone pair = trigonal planar geometry
2. 2 bonds + 1 lone pair = bent molecular geometry
B. Tetrahedral Electronic Geometry = 4 RHED
1. 4 bonds + 0 lone pair = tetrahedral molecular geometry
2. 3 bonds + 1 lone pair = trigonal pyramid molecular geometry
3. 2 bonds + 2 lone pair = bent molecular geometry
C. Trigonal Bipyramid Electronic Geometry = 5 RHED
1. 5 bonds + 0 lone pair = octahedral molecular geometry
2. 4 bonds + 1 lone pair = see-saw or distorted tetrahedral molecular geometry
3. 3 bonds + 2 lone pair = T-shaped molecular geometry
4. 2 bonds + 1 lone pair = linear molecular geometry
D. Octahedral Electronic Geometry = 6 RHED
1. 6 bonds + 0 lone pairs = Octahedral molecular geometry
2. 5 bonds + 1 lone pair = Square Pyramid molecular geometry
3. 4 bonds. + 2 lone pair = Square Planar molecular geometry
Transcription by James C. Gray MD FACOG

















Hello.nice to meet your report. haha.(sorry. Just kidding)
I’m a high school student in korea. I’m learnig chemistry, biology and other subject too.
I saw your writing nowyand i can understand this video,writing. So cool!!!!!
Asume!! I love this writing and three-dimensional image!
Thank you for upload useful informations.(if there is wrongunderstand just ignore them.!sorry😄)