VSEPR: Valence Shell Electron Pair Repulsion Theory–Part 1
by Janet Gray Coonce, MS
If you plan to view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges.
Review the notes after watching the video:
The two main principles of the VSEPR theory (Valence Shell Electron Pair Repulsion Theory) are:
1. Regions of high electron density (RHED) repel one another
2. Unshared RHED (“lone pairs”) take up a little more room than shared RHED.
Regions of high electron density (RHED) repel one another
There are two types of electron density regions:
1. Shared regions (purple balloons)
2. Unshared regions (yellow balloons)
Bond with 2 Shared Electrons
If there are 2 shared electrons between two atomic nuclei, then there is one region of high electron density (RHED).
Bond with 4 shared electrons
If there are 4 shared electrons between two atomic nuclei, then there is still only one REGION of high electron density (RHED). If there are 6 electrons between 2 nuclei, there is still just one RHED. If there is a resonance bond between 2 nuclei, there is still just one RHED.
Atom with 2 paired Electrons Occupying 1 RHED
UNSHARED pair or LONE PAIR of electrons are 2 non-bonded electrons on a central atom. In this illustration, the red ball represents the central atom, the yellow balloon represents the RHED occupied by the UNSHARED pair of electrons. The two dots represent the two valence electrons which are not participating in a bond, they just occupy a RHED and therefore take up space around the central atom. As a matter of fact the RHED occupied by UNSHARED electrons take up MORE space than RHED occupied by SHARED electrons.
Note of clarification: It is understood in the above illustration that the red ball representing the nucleus has other RHED other than the one illustrated. If the atom only had 2 electrons and only 1 RHED, the atom would be helium and both of the electrons would be in an s orbital (negative force field) surrounding the atom. In other words the nucleus would be in the center of the balloon and protected from forming any bonds. Helium is a noble gas. It is inert and does non reactive under normal conditions.
3 Dimensional Representation of Single Bond Geometry
If there are 2 shared electrons between two atomic nuclei, then there is one region of high electron density (RHED) and it represents a single bond between the atoms. A single bond is represented by a single line. It is a SINGLE RHED. That RHED is represented by the purple balloon. The single bond is represented by the single line between the atoms.
3 Dimensional Representation of Double Bond Geometry
If there are 4 shared electron between two atomic nuclei, then there is still only one REGION of high electron density (RHED). These electrons are shared in pairs and each represents a bond. Therefore 4 shared electrons represents 2 bonds, a double bond. Double bonds are represented by 2 lines drawn between the atoms. These two bonds are physically located within a SINGLE RHED. That RHED is represented by the purple balloon.
3 Dimensional Representation of Triple Bond Geometry
If there are 6 electrons between 2 nuclei, there is still just one RHED. The electrons are shared in pairs. There are 3 pairs of electrons being shared. This is a TRIPLE BOND and is represented by 3 parallel lines between the atoms. These three bonds and the 6 electrons are physically located within a SINGLE RHED. That RHED is represented by the purple balloon.
Resonance Bond in Benzene
A resonance bond is when there is a non-integer bond order between the pair of atoms. The bond order refers to the number of chemical bonds between a pair of atoms. When a pair of electrons is shared with other pairs of atoms (example bond between carbon atoms in the benzene ring) then the number of bonds per atomic pair will not be a whole number. This would be an example of a molecule with resonance bonding. A resonance bond is represented by a solid line and a dotted line between the atoms. The non-integer pairs of electrons are physically located within a SINGLE RHED. That RHED is represented by the purple balloon.
Linear Molecular Geometry (2 RHED)
If a central atom is bonded to 2 other atoms, the shared electrons between each of the atoms are going to repel one another. Electrons are negatively charged, like charged particles repel, therefore they are going to arrange themselves to get as far away from each other as possible. Therefore the bond angle between the bonds is 180◦. In other words, all three atoms and the 2 RHED between them will be arranged in a straight line. The molecular geometry is linear. The electronic geometry is linear.
Trigonal Planar Geometry (3 RHED)
If there are 3 RHED around a central atom repelling each other, we would predict the bond angle between the central atom and each of these regions to be 120◦ (360 degrees in a circle divided by 3). The electronic geometry and the molecular geometry is trigonal planar.
Trigonal Electronic Geometry with Bent Molecular Geometry (3 RHED)
If instead of 3 shared regions, there were 2 shared and 1 unshared pair of electrons, the repulsion forces would not be equal. The RHED of the unshared pair is more electro-negative than the 2 shared RHED. The electronic geometry is still trigonal planar because there 3 RHED. However, the molecular geometry is “bent” meaning that the bond angle is compressed and therefore less than 120◦. Sulfur dioxide (SO2) is an example of a molecule with this symmetry.
Tetrahedral Molecular and Electronic Geometry (4 RHED)
If there are 4 RHED with single bonds, then the electronic and molecular geometry is tetrahedral, not planar. The bond angles of 4 equal bonds surrounding a central atom are each 109.5◦. Methane (CH4) is an example of a molecule with tetrahedral molecular geometry.
Tetrahedral Electronic and Trigonal Pyramid Molecular Geometry (4 RHED)
Nitrogen is an example of an atom with a lone pair of electrons and 3 unpaired electrons each of which can be shared in a covalent bond. In ammonia gas, NH3, the nitrogen is covalently bonded with 3 hydrogen atoms. The electronic geometry is still tetrahedral because there are 4 separate RHED which are repelling each other. However there are only 4 atoms and the molecular geometry between the central nitrogen and the 3 hydrogen atoms is a trigonal pyriamid. Since the RHED occupied by the lone pair of electrons is more electronegative, the bond angles are predicted by the VSEPR theory to be bent to less than 109.5◦.
Trigonal Bipyramid Electronic and Molecular Geometry (5 RHED)
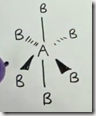
An example of this geometry is phosphorus pentachloride (PCl5). In this example, phosphorus has 5 RHED, each bonded to chlorine. It is not possible to arrange 5 regions around the central phosphorus atom with identical bond angles. This is a trigonal bipyrimid electronic and molecular structure. The bond angles are 120◦ between the 3 bonds on the equatorial plane. The bond angles are 90◦ between the bonds on the equatorial plane and those on the 2 bonds on the axial plane. The bond angle between the central phosphorus atom and the 2 hydrogen atoms on the axial plane is 180◦ , a straight line.
Model Representation of Trigonal Bipyramid Molecular Geometry (5 RHED)
It may help to visualize the planes using a model. The green balls represent the atoms on the equitorial plane. The blue balls the atoms on the axial plane. Notice that the blue balls are in a straight line with the central atom between them. The green balls are 120 degrees apart. The green balls are at a 90 degree angle to the axial plane.
2 Dimensional Diagram and 3 Dimensional Compared
In the 2 dimensional representation in the background, the solid wedge represents the green ball coming toward you. The dotted wedge represents the green ball going away from you. The angle from one blue ball to the other around the central atom is 180 degrees. The 90 degree angle between the blue and the green balls is labeled on the 2 dimensional model. The 120 degree angle between the green balls is also labeled on the 2 dimensional illustration.
Octahedral Geometry (6 RHED) Illustrated in 2 and 3 Dimensions
An example of a molecule with 6 RHED is Sulfur hexafluoride (SF6). Visually compare and understand the second 2 dimensional drawing of the 3 dimensional models. The balloons in the first image represent the electronic geometry of the 6 RHED. The 3rd image is of a molecular model which demonstrates the relationship of the atoms, the molecular geometry. In this case both the electronic and the molecular geometry is octahedral. The second image demonstrates how to illustrate this geometry in 2 dimensions.
The VSEPR (valence shell electron pair repulsion) theory predicts both the molecular and the electronic geometry based on the chemical formula.
Electronic Geometry: the shape of the arrangement of ALL RHED surrounding the central atom
Molecular Geometry: The shape of the arrangement of atomic nuclei in the molecule
If you know the formula of a molecule and can draw the Lewis dot structure of that molecule, then you can predict both the electronic and the molecular geometry of that molecule.
Electronic Geometry (Trigonal Planar) of Sulfur dioxide (SO2)
Molecular Geometry (Trigonal Planar) of Sulfur dioxide (SO2) is Bent (< 120 degrees)
Illustrating how the lone unpaired electrons repel the less negative electrons being shared in the 2 bonds and bend the bond angles to less than 120 degrees.
Part II will begin at 9 minutes 48 seconds into the YouTube video.
Transcribed by James C. Gray MD FACOG