Hybridization Geometries & Bond Angles
Chemistry Video by Janet Gray Coonce MS
If you plan to view the video on your cell phone, consider your data plan and whether you should wait until you have a WiFi connection to avoid cellular charges.
Review the notes after viewing the video:
If a central atom has 2 sigma ‘s’ bonds and no lone pairs then the molecular shape is linear with a predicted 180◦ bond angle, in other words the orbitals are arranged in a straight line with the central atom. This central atom is said to be sp hybridized. This illustration shows 2 sp hybridized orbitals getting as far apart as possible. The geometry is linear.
What do I mean by sp?
An s orbital is in the shape of a sphere and a p orbital is in the shape of a dumbbell. These shapes define the probability of finding an electron within that space. In an s orbital the electron can be found in a sphere surrounding the nucleus. In a p orbital the electron can be found on opposite sides of the central nucleus. In this illustration, the probability of finding an electron in the py orbital is defined by a dumbbell shape with the bulbs of the dumbbell above and below the central atom.
There are 3 orientations of the p orbital. One on the x, y, and z axis. In this illustration they are designated px, py, and pz.
Here colored play dough is used to create a 3 dimensional representation of these orbitals. The s orbital is represented by the blue sphere and the p orbitals are represented by the yellow dumbbell shapes.
Here the blue s and yellow px orbitals were mixed to form 2 green hybridized sp orbitals. These two sp hybridized orbitals will orient themselves so that they will be as far apart as possible. They will have a linear arrangement, a 180◦ bond angle.
Now the play dough orbitals are assembled. The empty p orbitals, represented by yellow, are perpendicular to each other in the same plane. The sp hybridized orbitals, represented by the green are centered and perpendicular to the plane of the yellow p orbitals.
Example: Ethyne (acetylene) C2H2
Each carbon in the molecule acetylene can be represented by the electronic orbital configuration of this model. See the video and blog "Sigma and Pi Bonds" to see how these orbitals form a triple bond.

A sigma bond occurs when the electron overlap occurs between the nuclei of the atoms.
A single bond is a sigma bond
A double bond is a sigma bond and a pi bond.
A triple bond is a sigma bond and 2 pi bonds.
Trigonal Planar Molecular Geometry, Example Aluminum Bromide
When a central atom is involved with 3 sigma bonds, the orbitals will align themselves as far apart as possible around the central nucleus as illustrated here. The bond angles are 120◦. There is one s orbital and 2 p orbitals. The orbitals are sp2 hybridized, the geometry is trigonal planar. It is all on the same plane. An example would be AlBr3.
Bent Molecular Geometry, Trigonal Planar Electron Geometry.
If the atom has 2 sigma bonds and a lone pair of electrons, it is still sp2 hybridized. There are 3 electron regions in the same plane with predicted bond angle of slightly less than 120◦. This “bent” sp2 hybridization configuration is predicted due to the higher electronegativity of the lone pair of electrons compared to the sigma bonds. The electronegativity of the lone pair of electrons will repel and therefore bend the angle between the sigma bonds to less than 120 degrees. This is the effect of valence shell electron pair repulsion (VSEPR). Even with a lone electron pair and 2 sigma bonds, there are 3 electron regions, it is still referred to as sp2 hybridization. An example of a molecule with 2 sigma bonds and a lone pair of electrons is S02.
Tetrahedral Molecular Geometry
With 4 sigma bonds and no lone pairs there are 4 electron regions and the molecular shape is tetrahedral. The predicted bond angle is 109.5◦. This central atom is sp3 hybridized. Methane (CH4) is an example of a molecule with sp3 hybridization with 4 sigma bonds.
Trigonal Pyramid Molecular Geometry
 This atom has 3 sigma bonds and a lone pair. There are 4 areas of electron density. It is sp3 hybridized and the predicted bond angle is less than 109.5◦. It has a trigonal pyramid geometry. An example is NH3, ammonia gas.
This atom has 3 sigma bonds and a lone pair. There are 4 areas of electron density. It is sp3 hybridized and the predicted bond angle is less than 109.5◦. It has a trigonal pyramid geometry. An example is NH3, ammonia gas.
If there are 2 lone pair of electrons and 2 sigma bonds there are still 4 areas of electron density. It is sp3 hybridized. A “bent” molecular shape is predicted due to the electronegative repulsion by the 2 lone pair of electrons as predicted by the VSEPR theory. Therefore the predicted bond angle is less than 109.5◦. An example is H20.
Trigonal Bipyramid Molecular Geometry
PCl5 gas is an example of a molecule with 5 dsp3 orbitals with trigonal bipyramid geometry. It is not geometrically possible to arrange 5 bonds equal distant around a central atom. In this model I use two colors to represent the chloride atoms. On the equatorial plane the chloride atoms are dark green. There are 3 bonds on the equatorial plan and the bond angles are equal to 120◦. On the axial plane the chloride atoms are colored red. The bond angle is 90◦ between the atoms on the axial plane and those on the equatorial plane.
See-Saw Molecular Geometry
SF4 gas is an example of a molecule with 5 dsp3 orbitals but one of the orbitals contain a lone pair of electrons. The bond angle is still 90◦ between the atoms on the axial plane (red) and those on the equatorial plane (dark green). The angle between the sigma bonds on the equatorial plane (dark green atoms) are bent and therefore are less than 120◦.
T-Shaped Molecular Geometry
ClF3 is a T-shaped dsp3 hybridized molecule. It has 3 sigma bonds and 2 pair of lone electrons. The lone electrons are in dsp3 hybridized orbitals on the equatorial plane. The bond angle is still 90◦ between the atoms on the axial plane (red) and those on the equatorial plane (dark green). All 4 atoms in chlorine trifluoride are halogens from group VIIA in the periodic table. All of them need only one electron to complete the octet rule.
Rocket science factoid: Although ClF3 is “happy” that the valence shell of each atom is filled, all of them would be much happier if they could each get an electron from something other than another member of the halogen group. A halogen would much prefer a metal to share its electrons. Chlorine trifluoride is highly reactive and was considered to be used in rocket fuel as an oxidant (removes electrons from another substance). It was pressurized and put in metal containers lined with metal fluoride. However, it was too reactive. If conditions (such as heat) caused the protectant metal fluoride coating to come off, the atoms in the chlorine trifluoride molecule will steal electrons from (react with) the metal container in which the pressurized liquid ClF3 was stored. In other words it could potentially burn and blow up the container!
Tetrahedral Electrical Geometry but Linear Molecular Geometry
If a dsp3 central atom has 2 sigma bonds and 3 pairs of lone electrons, it will have linear geometry. The bond angle is 180◦ which is a straight line. XeF2 is an example of a molecule with this configuration. It is a strong fluorinating agent.
Octahedral Molecular Geometry
A central atom with 6 sigma bonds is d2sp3 hybridized and is octahedral in shape. An example is SF6. The central atom has 2d + 1s + 3p = 6 hybridized orbitals. The bond angles are all equal at 90◦.
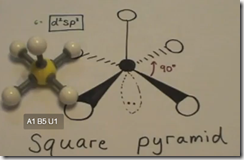
Octahedral Electrical Geometry with Square Pyramid Molecular Geometry
If one of the sigma bonds are replaced with a lone pair of electrons, the molecule would have a square pyramid geometry. BrF5 is an example. Like ClF3 it is also an interhalogen compound and very reactive.
Square Planar Molecular Geometry
If 2 of the sigma bonds are replaced with a lone pair of electrons, the molecule is still d2sp3 hybridized but with a square planar geometry. XeF4 is an example.
Tetrahedral and Square Planar Geometry Compared
Contrast the sp3 hybridized tetrahedral model on the left with the d2sp3 hybridized square planar model on the right. The geometry is completely different.
Now lets use the valence bond theory to make predictions about these molecules. The central carbon has 4 electron regions surrounding it so we know it is tetrahedral and sp3 hybridized. We expect a bond angle of 109.5◦. There are no empty p orbitals because all 3 p orbitals were used to hybridize into the tetrahedral molecular shape.
The carbons in this Lewis dot structure have 3 bonds 120◦ apart and are sp2 hybridized. There are 3 sigma bonds and one pi bond. The pi bond forms from the p orbital above and below the plane of these atoms.
Each carbon in this molecule have 2 sp orbitals in a linear geometry with 2 overlapping p orbitals connecting the carbons to each other. So each carbon is sp linear and the overall molecule is linear.
Summary:
Hybridized orbitals geometry Example
sp linear (180◦) C2H2 = acetylene
sp2 trigonal planar AlBr3 = aluminum tribromide
sp3 tetrahedral planar (109.5◦) CH4 = methane
dsp3 trigonal bipyramid (90◦, 120◦) PCl5
= phosphorus pentachloride
d2sp3 octahedral (90◦) SF6 = Sulfur hexafluoride
Transcription by James C. Gray MD FACOG





















Wow! This helped me to understand the concept of hybridization in an amazing way, Thanks to the author and continue!